Type 1 diabetes results from impaired central and peripheral tolerance mechanisms. Beta-cell antigen-reactive T cells escape negative selection in the thymus and regulation/deletion in the periphery. While the role and therapeutic potential of professional antigen-presenting cells and regulatory T cells in maintaining peripheral tolerance in type 1 diabetes is known, non-professional antigen presenting stromal cells residing in lymph nodes remain understudied. For maintaining and therapeutically promoting peripheral tolerance to self-antigens such as type 1 diabetes-relevant ones, these stromal cells present unique advantages over other antigen-presenting cells due to their lower costimulatory to coinhibitory molecules ratio, which remains stable during inflammation unlike activated professional antigen-presenting cells.
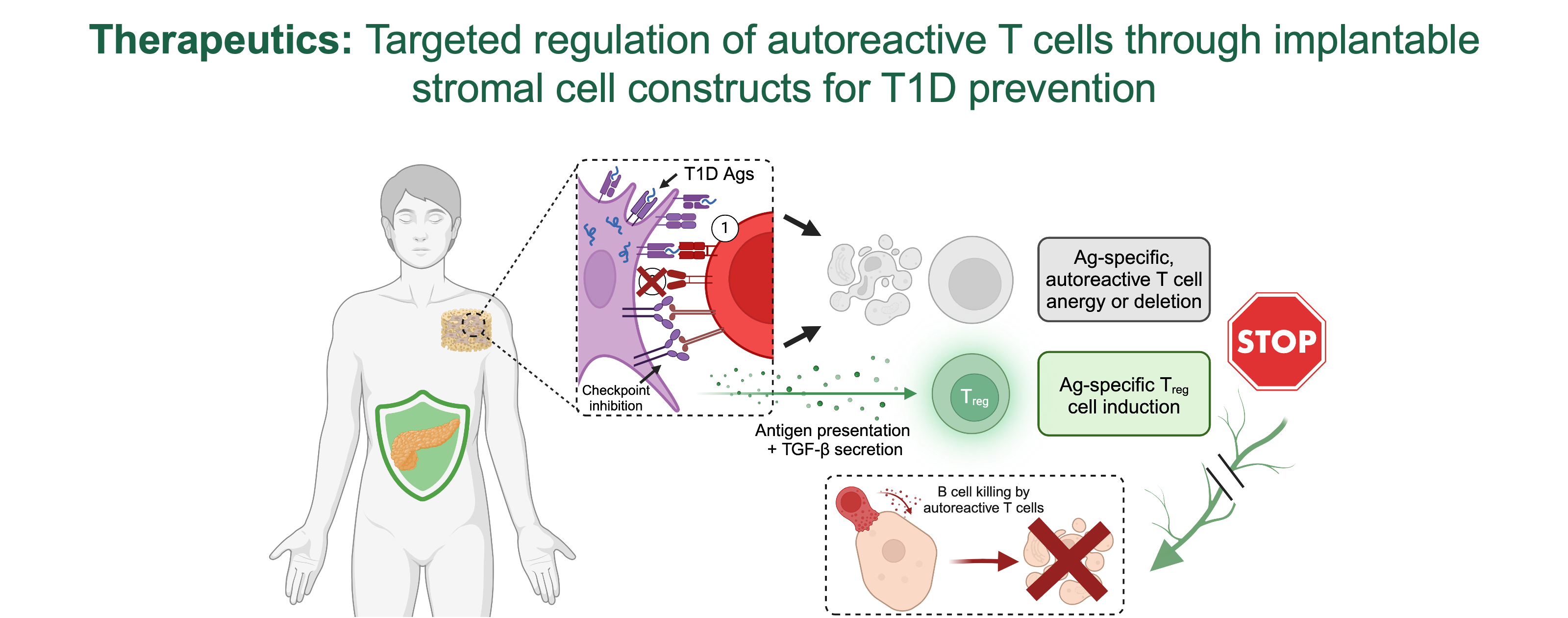
Our focus is on fibroblastic reticular cells (FRCs) which construct the lymph node stromal scaffold crucial for lymph node expansion during immune responses. FRC presentation of artificial antigens to specific T cells in lymph nodes lead to T cell deletion. Our studies showed that in the non-obese diabetic mouse model of type 1 diabetes, and in pancreatic lymph nodes from pancreatic organ donors with type 1 diabetes, the expression of the autoantigen insulin, the relative frequency of FRCs, and the FRC reticular remodeling properties are decreased. Thus, we hypothesize that peripheral expression and presentation of tissue-specific antigens by FRCs to autoreactive T cells may contribute to peripheral tolerance. To test our hypothesis that lymph node FRCs contribute to peripheral tolerance in type 1 diabetes, we have developed new mouse models to inhibit or enhance the capability of lymph node FRCs to express and present self and type 1 diabetes-relevant antigens. In Aim 1, we will evaluate the impact of these modifications on autoimmunity.

Additionally, to test FRCs’ ability to target autoreactive T cells and reduce autoimmunity, we propose innovative in vitro co-culture models with HLA-matched human cells in Aim 2. These studies are based on our preliminary data using murine FRCs. We demonstrated that (i) we can engineer FRC reticula that recapitulate FRC reticula organization and phenotype in lymph nodes, and (ii) genetically engineered FRC reticula to express and present type 1 diabetes-relevant antigens promote specific T cell anergy and regulatory T cells induction and expansion in vitro. Here, we will test the capability of human lymph node-derived FRCs to provide similar immunomodulatory effects on HLA-matched human T cells leading to decreased cytotoxicity on primary and stem cell-derived HLA-matched b-cells for translatability.
While the proposed studies are focused on the specific role and therapeutic application of lymph node FRCs in autoimmunity, our innovative immunoengineering approaches can be applied to the study of other lymphoid and mesenchymal stromal cells. The interdisciplinary nature of our MPI team positions us uniquely to conduct these studies.