Type 1 diabetes (T1D) is an autoimmune disease that leads to selective destruction of insulin-secreting pancreatic β cells and lifelong dependence on exogenous insulin supplementation. In the US, 1.84 million people have T1D and 5 million people are expected to be diagnosed by 2050.
Allogeneic β cell replacement through intrahepatic islet transplantation leads to better metabolic control, fewer long-term complications, and improved quality of life in patients with T1D as compared to exogenous insulin supplementation. The field of β cell replacement has recently benefited from major progress that addressed several limitations of the procedure for treatment of T1D. These recent advances have increased the hope of the millions of patients with T1D for a widely available cure. However, the continuing need for chronic systemic immunosuppression to prevent allorejection and recurrence of autoimmunity restricts the application of β cell replacement to the most severe cases of T1D due to a multitude of unavoidable side effects.
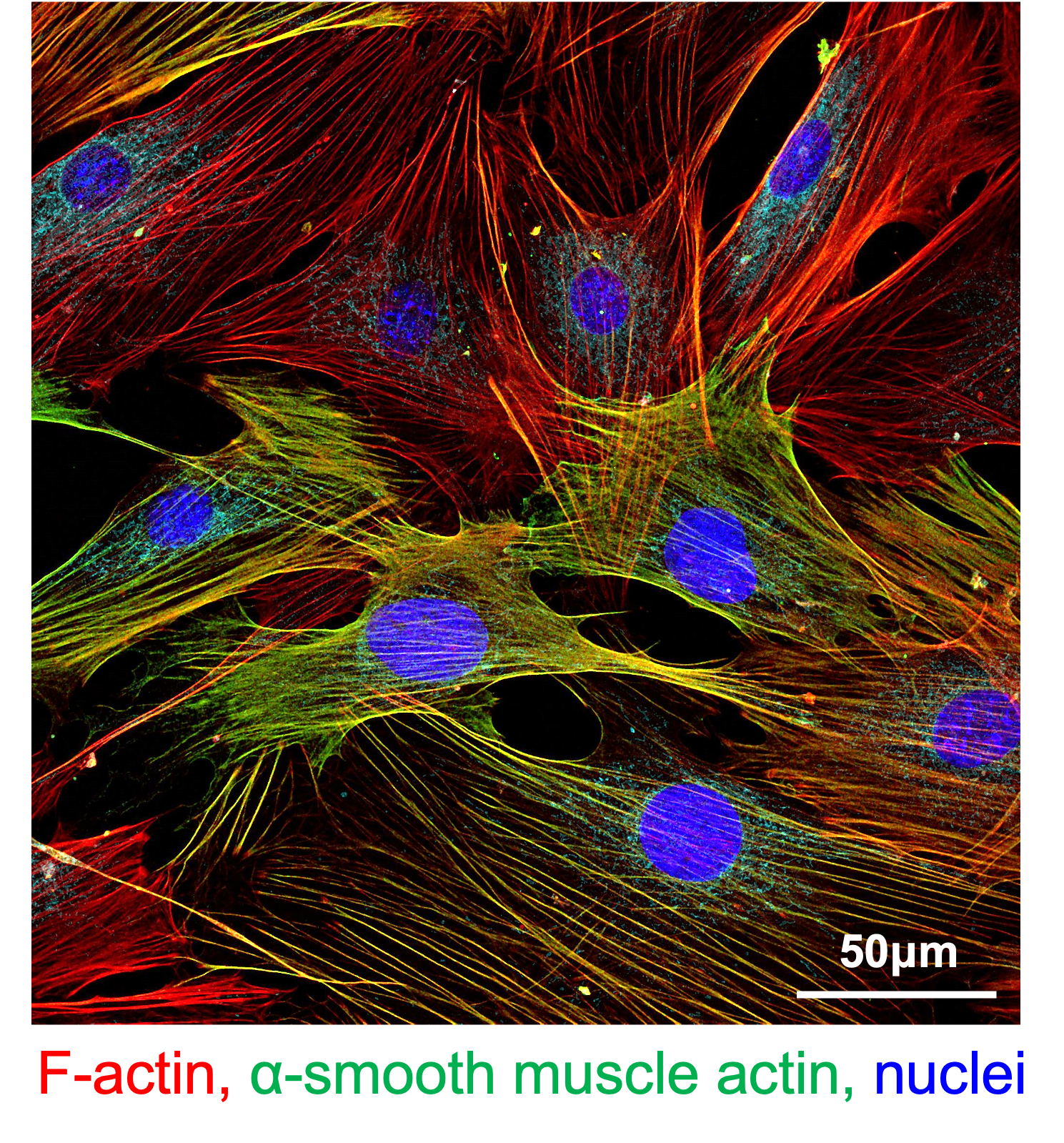
Different strategies have been tested to achieve safer and more widely applicable β cell replacement for patients with T1D. Localized immunomodulation in the islet graft is enabled by extrahepatic transplantation in clinically-applicable confined sites such as the omental, intramuscular, and the subcutaneous sites. The clinically approved co-stimulation blocker abatacept (CTLA4Ig, human IgG1 subclass) blocks CD80/86 signaling required for T cell activation and promotes allograft survival in both preclinical models and in patients. Similarly, anti-CD40L antibodies were also effective in preclinical and clinical settings. However, systemic injection of these co-stimulatory blocking antibodies and immunomodulatory biologics in general is associated with adverse effects. This shortcoming can be addressed by non-systemic, localized, sustained delivery. Alternative to local immunosuppression, co-delivery of islets with immunomodulatory cells has demonstrated beneficial effects in islet transplantation by decreasing the need for systemic immunosuppression and inducing tolerance. Co-transplantation with tolerogenic non-professional stromal antigen-presenting fibroblastic reticular cells (FRCs) can also decrease alloreactive T cell activation and promote long-term allograft survival. These cells are a subpopulation of lymph node stromal cells that play key roles in peripheral tolerance through different mechanisms. We demonstrated that FRCs can present selected antigens to specific T cells inducing anergy and regulation in vitro.
 We hypothesize that localized and sustained release of selected human IgG1-based co-stimulatory blocking biologics by human IgG1-capturing degradable hydrogels co-delivered with islets in a confined site will prevent alloreactive T cell activation to prolong allograft survival in mice (aim 1).
We hypothesize that localized and sustained release of selected human IgG1-based co-stimulatory blocking biologics by human IgG1-capturing degradable hydrogels co-delivered with islets in a confined site will prevent alloreactive T cell activation to prolong allograft survival in mice (aim 1).
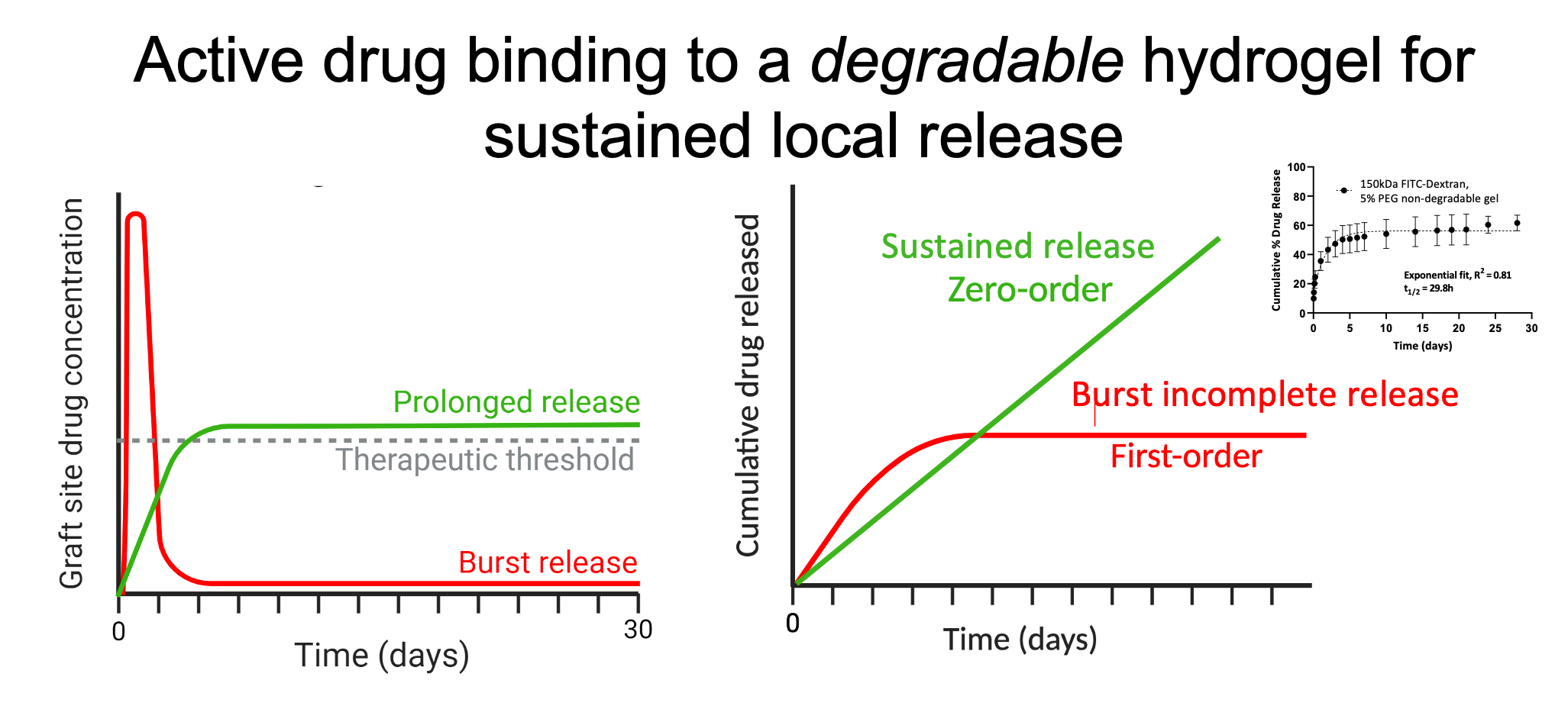
We also hypothesize that co-transplantation of pre-conditioned, antigen-primed, syngeneic fibroblastic reticular cells alongside allogeneic cells will decrease activation and tolerize recipient alloreactive T cells to improve murine allograft survival (aim 2). In aim 1, we will optimize the design of biologics-binding degradable hydrogels to provide sustained release of abatacept and anti-CD40L and test therapeutic efficacy in vitro and in vivo in murine allograft models. We will determine the dose of IgG1-binding peptide, modified for covalent binding to degradable polyethylene glycol hydrogels, that prevents burst release and maintains abatacept and anti-CD40L at therapeutically effective local levels (e.g., low nanomolar concentrations representing ~10x IC50 values).
In aim 2, the protocol for FRC co-transplantation will be optimized by determining the effects of IFNγ and antigen pre-conditioning on C57BL/6-derived FRC phenotype, in vitro efficacy of FRCs in attenuating alloresponses, and in vivo retention, viability, and in vivo efficacy of FRCs in attenuating alloresponse.
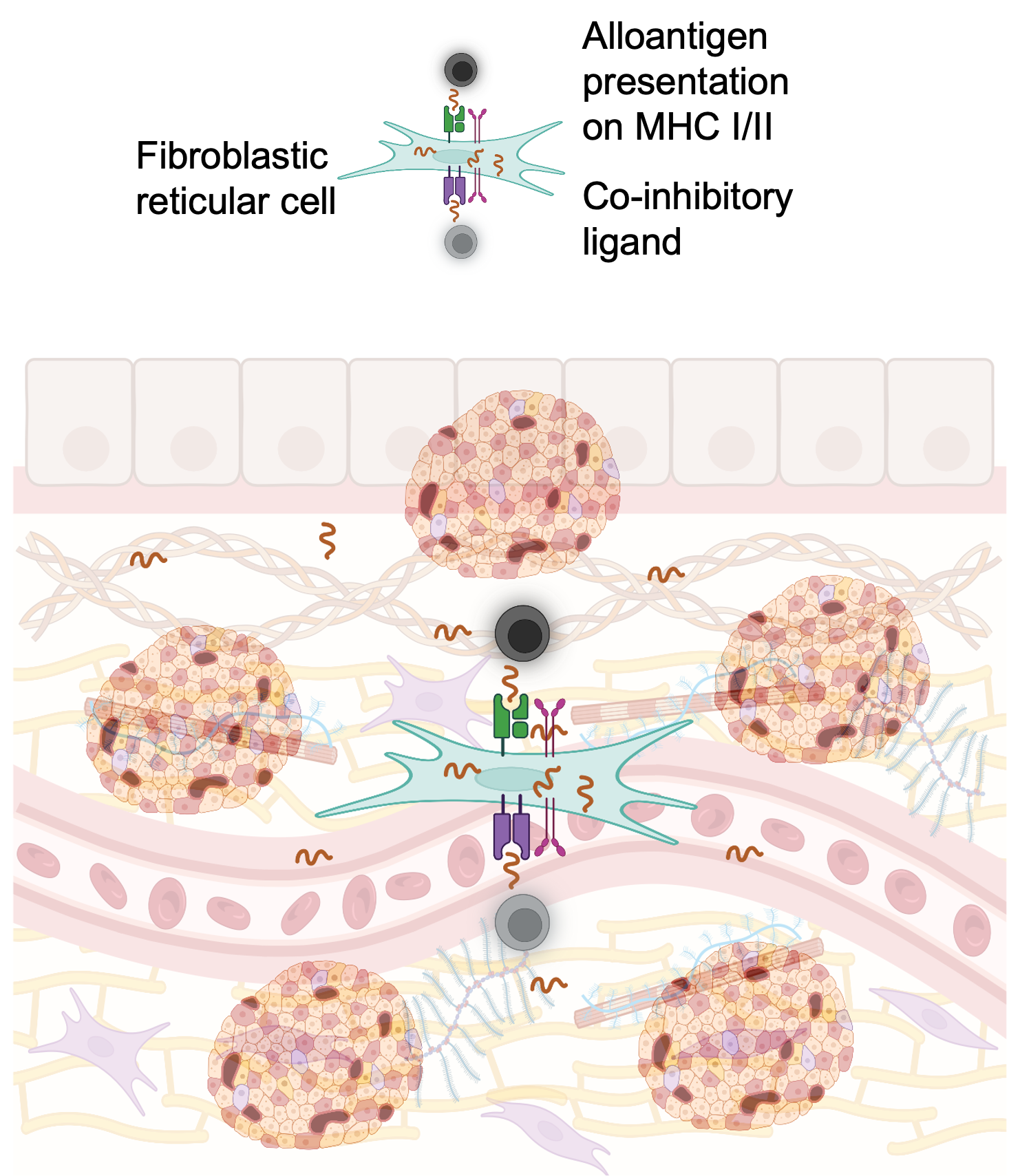
Our approaches are applicable to islets as well as stem cell-derived beta cell replacements, novel extrahepatic confined transplantation sites, and other antibody-based drugs. Furthermore, our approaches could be exploited by other investigators to enhance current strategies to improve beta cell replacement therapies through islet transplantation, including those that have reached the clinical translation stage.



 We hypothesize that localized and sustained release of selected human IgG1-based co-stimulatory blocking biologics by human IgG1-capturing degradable hydrogels co-delivered with islets in a confined site will prevent alloreactive T cell activation to prolong allograft survival in mice (aim 1).
We hypothesize that localized and sustained release of selected human IgG1-based co-stimulatory blocking biologics by human IgG1-capturing degradable hydrogels co-delivered with islets in a confined site will prevent alloreactive T cell activation to prolong allograft survival in mice (aim 1).

