In the United States, 1.84 million people have type 1 diabetes with 21% increase in diagnosis between 2001 and 2009, and 5 million people expected to be diagnosed by 2050.
Patients with established type 1 diabetes can benefit from beta cell replacement through transplantation of islets obtained from organ donors or stem cell-derived islets. This procedure restores a patient’s ability to produce their own insulin, which eliminates the need for insulin injections, improves metabolic control and quality of life, and reduces long-term complications. However, the procedure is currently performed only in patients with severe type 1 diabetes due to the unavoidable side effects of the chronic systemic immunosuppression needed to prevent islet rejection and recurrence of autoimmunity.
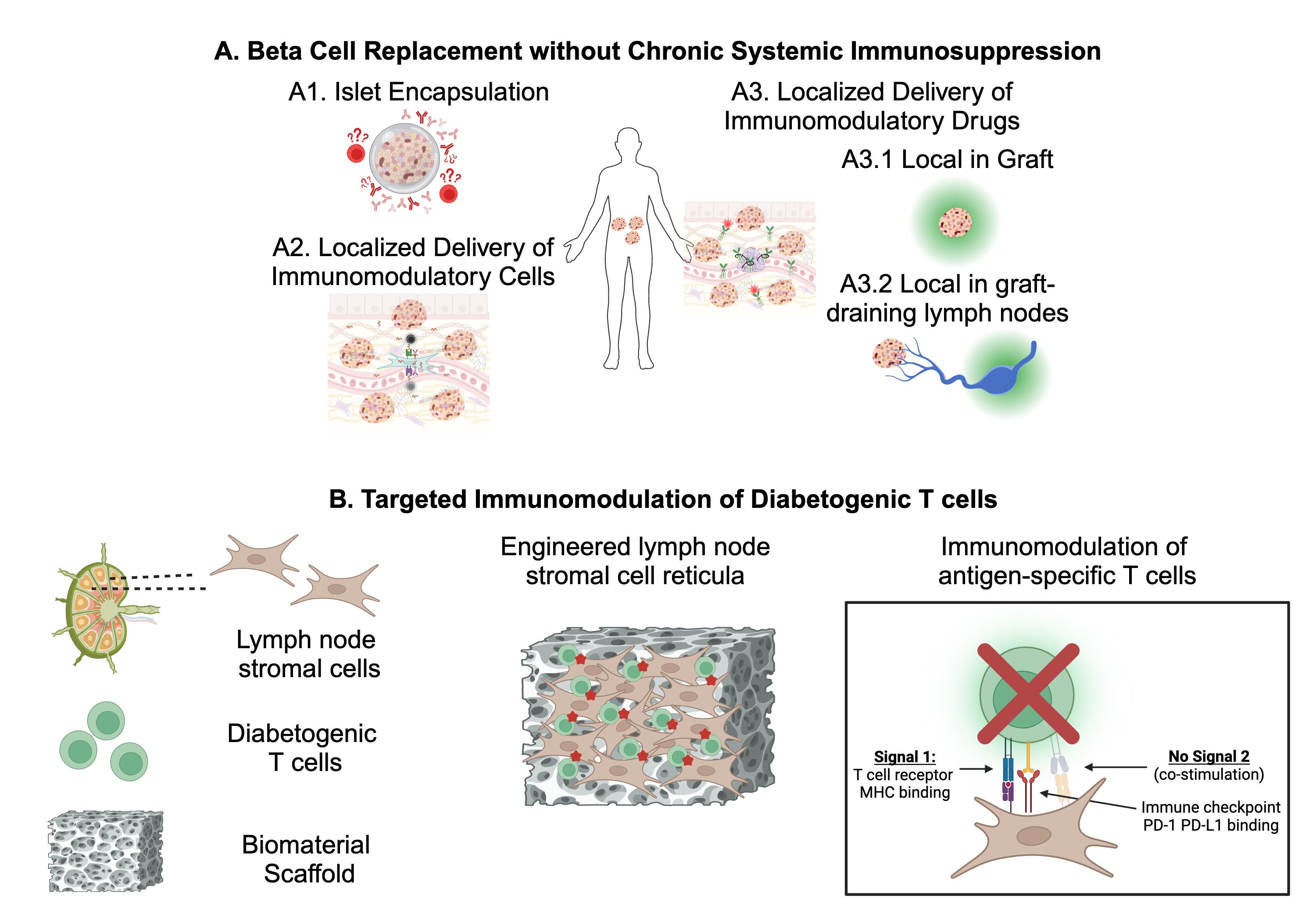
In my lab, we are evaluating innovative approaches for encapsulation using our innovative platform for islet microencapsulation through conformal coating, (Fig.1A1) and localized immunomodulation using tolerogenic stromal cells (Fig.1A2) and/or biomaterials (Fig.1A3) to prevent islet rejection and recurrence of autoimmunity while sparing patients’ overall immunity. In doing so, these approaches will increase the safety and accessibility of islet transplantation for patients with established type 1 diabetes.
We are testing these approaches in preclinical animal models of type 1 diabetes, and the approaches we are testing have high clinical translatability since the coating biomaterials and the drugs we are testing are clinically approved, and the stromal cells can be isolated from patients with type 1 diabetes through minimally invasive procedures.
If successful, our approach can address the remaining challenges of β cell replacement for patients with type 1 diabetes by eliminating the need for chronic systemic immunosuppression and can be used in combination with some of the most innovative approaches, including transplantation of stem cell derived β cells and extrahepatic islet transplantation.
Dysregulation in immune tolerance mechanisms leading to unbridled reactivity to self-antigens precipitates tissue damage, functional loss, and multi-organ afflictions and culminate in autoimmune diseases, whose societal impact, exemplified by conditions such as type 1 diabetes, underscores the need for innovative approaches capable of safely and selectively modulating immune cells responsible for driving pathogenic processes. Our research endeavors to advance our comprehension of lymph nodes as focal points for immune tolerance induction and aims to assess novel therapeutic strategies for autoimmune diseases, leveraging engineered stromal cells as agents for promoting tolerance (Fig.1B). By elucidating the intricacies of lymph node-mediated immune tolerance and evaluating innovative therapies, our project seeks to contribute substantively to the development of effective interventions for preventing and treating autoimmune diseases.