Type 1 diabetes is an autoimmune disease that leads to selective destruction of insulin-secreting pancreatic beta cells and lifelong dependence on exogenous insulin supplementation. In the United States, type 1 diabetes affects 1.84 million people, increased 21% in diagnosis between 2001 and 2009, and is projected to affect 5 million people by 2050. Allogeneic beta cell replacement through intrahepatic islet transplantation leads to improved metabolic control, quality of life, and decreased levels of long-term complications in patients with type 1 diabetes compared to exogenous insulin supplementation. This is largely due to the capability of transplanted islets to re-establish physiological glucose-stimulated insulin secretion. Recent clinical advances in islet transplantation have increased the hope of the millions of patients with type 1 diabetes for a widely available cure. However, all this progress notwithstanding, the need for chronic systemic immunosuppression to prevent allorejection and recurrence of autoimmunity is still restricting the applicability of beta cell replacement procedures only to the most severe cases of type 1 diabetes due to the multitude of unavoidable side effects.
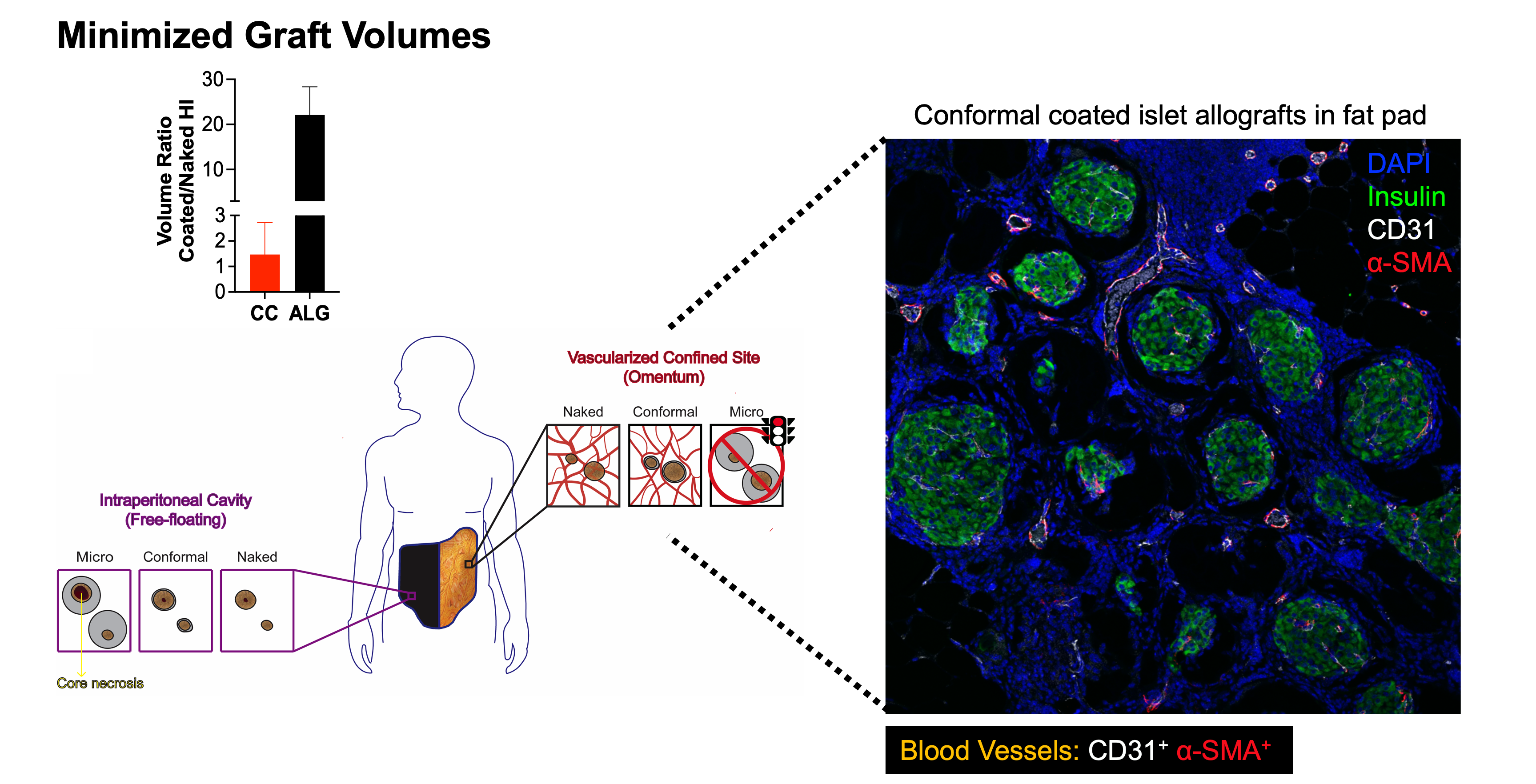
Islet immunoisolation through encapsulation with permselective biomaterials is a viable option to allow safer and more widely applicable beta cell replacement for patients with type 1 diabetes. The main challenges associated with transplantation of encapsulated islets are (1) their large capsule size, which delays glucose sensing and insulin release and results in an inability to achieve the same level of metabolic control as that provided by unencapsulated islets, and (2) their large graft volumes, which prevent transplantation in retrievable, confined, and well-vascularized sites. We developed an innovative microencapsulation platform that achieves conformal coating of islets, including ones derived from stem cells, within biocompatible, stable, and clinically applicable hydrogels that are only 10-20 µm thick.
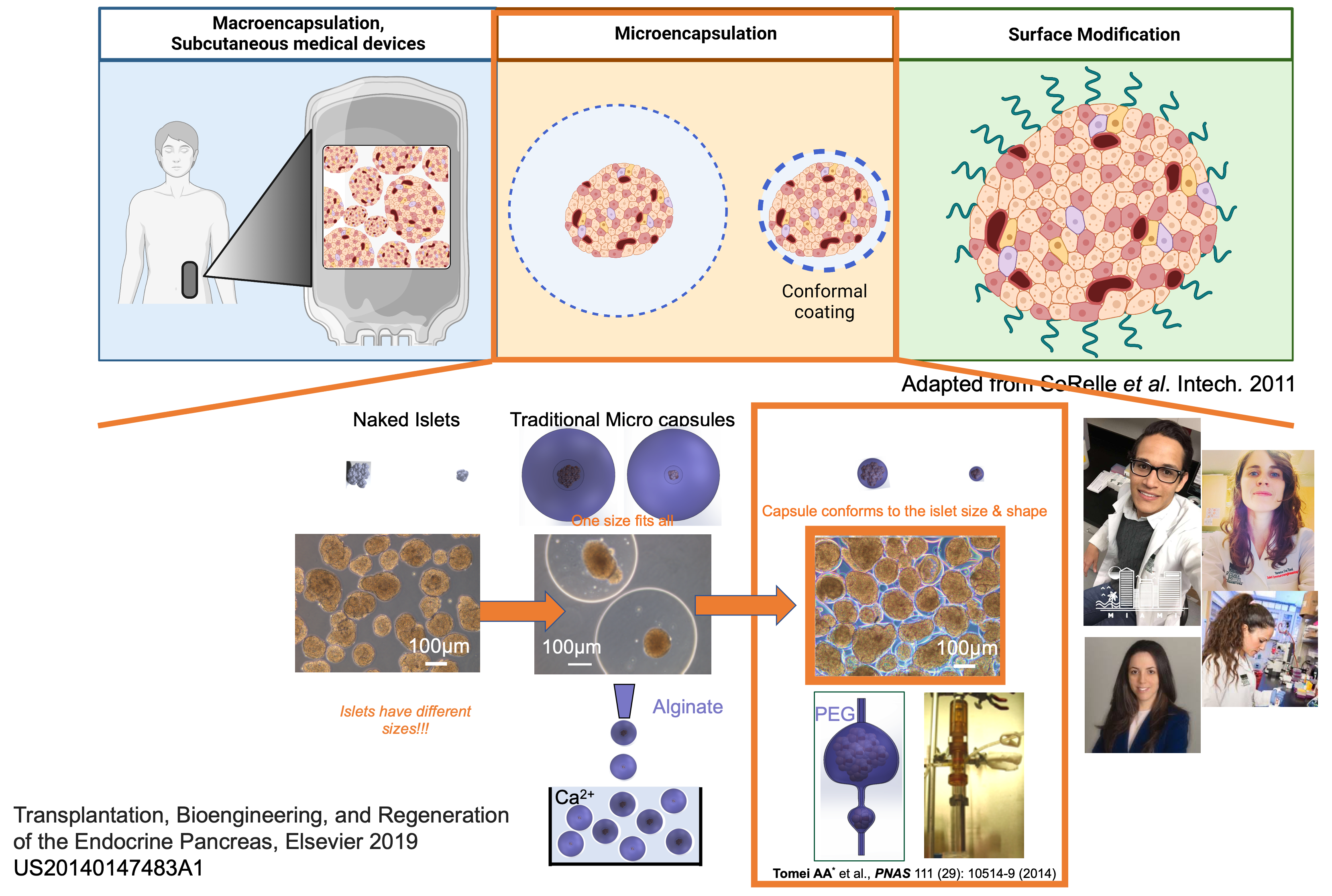
This conformal coating addresses limitations of traditional islet encapsulation platforms and safety as demonstrated in non-human primate models of type 1 diabetes. However, conformally coated grafts are still hindered by alloreactive T cell activation by alloantigen shedding through permeable hydrogel capsules and can benefit from localized immunomodulatory therapies to improve their performance for clinical translation. Thus, we propose to co-transplant them with our innovative nanomaterial platform to achieve targeted and sustained release of drugs that decrease alloreactive T cell activation in the site of transplantation (aim 1). As an alternative and innovative approach, we also propose to integrate islet conformal coating with co-delivery of tolerogenic stromal cells that can present shed alloantigens to specific T cells, thereby inducing anergy and deletion in the transplant site (aim 2). We will test these combination therapies in murine and rat islet allograft models of type 1 diabetes in clinically applicable confined sites, uniquely enabled by the small volumes of conformal coated grafts in aims 1 and 2. In aim 3, we will test the most promising immunomodulatory approach in non-human primate islet allograft models and with human islets in a humanized mouse model.